Contamination Control
CONTAMINATION CONTROL:
In cleanrooms and controlled environments it is essential to minimize the risks of contamination for sensitive products and for the personel.
Contamination control as well as the control of unacceptable changes in the environment (humidity, temperature, air-flow) requires the highest standard of diligence.
The basis of good contamination control:

Contamination control measures are one of the basic requirements for the manufacture of medicinal products. Pharmaceuticals manufacturing requires a defined and valid system to avoid contamination, as well as a clear disaster strategy to handle any microbiological deviations.
Normally, one would expect that awareness of these activities is programmed into everyone working in the Good Manufacturing Practice (GMP) field.
Steris: A global leader in infection prevention and contamination control
STERIS has been providing leading Science and Solutions to the Life Sciences industry for more than 100 years. Their participation and reach in this field gives them a deep understanding of the high standards and important mission which challenge our Customers. Throughout their history, they have advanced the Science of infection control, cleaning and sterilization by delivering new technologies and Services which solve problems for our Customers. Their advanced portfolio of Formulated Chemistries and Capital Equipment combined with their global team of respected industry professionals allow us to tackle difficult problems, find Solutions and provide world class Service.
STERIS Decontamination and Sterilization Products:
Whether you are a researcher or a manufacturer, STERIS has solutions to help prevent contamination at virtually every critical point in your process. Across the globe, STERIS provide solutions to help create and maintain clean and sterile environments through our equipment, formulated cleaning chemistries, and service solutions. To learn more about how STERIS can help you become more productive, manage your risk, and reduce operating costs see their list of solutions below.
Detergents for Laboratory, Pharmaceutical & Manufacturing Environments
- Pharmaceutical Detergents and Cleaners
- Glassware Washing Detergents
- Cage Washing Detergents
Surface Disinfectants & Cleaners
- Spor-Klenz® Sporicidal Disinfectants & Sterilants
- Sterile Hydrogen Peroxide & Oxidizing Cleaners
- Pharmaceutical Disinfectants
- Sterile Alcohols
Biological & Chemical Indicators
- Biological Indicators for Sterilization
- Chemical Indicators for Sterilization
Barrier Products
- Cleanroom Component Prep
- GMP® Equipment Covers
- Cleanroom Tools and Supplies
- Cleanroom Apparel and Barrier Product Solutions
- Stopper Bowl Covers
Decontamination Equipment
- Pure Steam & Water Systems
- Steam Sterilizers
- VHP Sterilization & Biodecontamination
- Glassware Washers and Dryers
Steam Sterilizers
- Steam Sterilizers
Modular Cleanroom Solutions for Pharmaceutical and Lab Environments
Hand Hygiene
- Sterile Alcohol Hand Rinses and Handrubs
- Soaps
- Lotions
- Soap Dispensers and Adapters
VHP Sterilization & Biodecontamination:
STERIS vaporized hydrogen peroxide (VHP) biodecontamination systems use their patented VHP “dry process” technology and proprietary Vaprox Hydrogen Peroxide Sterilants to support biotechnology, pharmaceutical manufacturing, and related industries.
The VHP sterilization equipment uses hydrogen peroxide vapor as a broad spectrum anti-microbial. It evenly distributes VHP throughout the enclosure, providing efficacy against viruses, bacteria, yeasts, and bacterial spores.
This “dry process” separates STERIS from other companies because it doesn’t leave potentially damaging condensation or toxic residue on surfaces. This means that STERIS VHP biodecontamination systems provide a wide range of material compatibility.
Central/ Integrated VHP system for Pharma Industry:
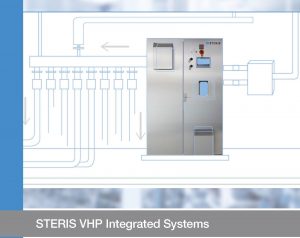
A pharmaceutical-grade VHP system for fast, continuous, large-enclosure biodecontaminationin applications such as pharmaceutical, aseptic filling, and those requiring integration into an AHS/HVAC system.
Product Overview:

STERIS’ VHP® M1000-T4 is a high capacity, safe & environmentally friendly method to allow for easy & efficient daily decontamination of large spaces.
STERIS’ VHP M1000-T Biodecontamination System utilizes Vaprox® Sterilant (EPA Reg. No. 58779-4) as a means of achieving dry, low temperature sterilization within large sealed enclosures such as cleanrooms and filling lines. The dry, gaseous process consists of four distinct phases:
Dehumidification – Humidity is removed from the room space via an integrated desiccant system. This is done to ensure that a true, dry biodecontamination process is achieved.
Conditioning – Vaprox is rapidly injected by the generator and converted into a dry vapor that quickly raises the level of hydrogen peroxide to an effective concentration.
Biodecontamination – Hydrogen peroxide vapor concentrations are maintained at the target concentration level to provide an effective kill of micro-organisms in the room space.
Aeration – The generator stops injecting Vaprox and accelerates the breakdown of vapor into its environmentally friendly base elements of water vapor and oxygen. Aeration continues until hydrogen peroxide is reduced to an acceptable level and the room is commissioned for regular use.
WHY VHP M1000-T4 BIODECONTAMINATION SYSTEMS?
Productivity – Provides continuous high capacity decontamination of large spaces, >20,000ft3, such as clean rooms, production filling line isolators, pass-through chambers, etc.
Ease of Use – Can be integrated with building automation systems.
Flexibility – Can be conveniently integrated into pass-through chamber or aseptic filling systems or used to decontaminate entire suites or rooms in sequence.
Efficacy – The gaseous decontamination process is effective against a wide variety of microorganisms and has a broad range of material compatibility.
Green – Provides a non-toxic and environmentally friendly alternative to other decontamination processes. Vaporized Hydrogen Peroxide breaks down to water vapor and Oxygen.
Secure – Write text saying Optional 21 CFR Part 11 data security package enables audit trail electronic data capture, user administration, and other features for use and validation.
Connectivity – Optional Anybus modules allow for easy integration into system network via EtherNet/IP, Modbus TCP, and PROFIBUS communication protocols.
